My students are also blogging here:
My students get a full exposure to the Sean Carroll perspective in his book, Endless Forms Most Beautiful, and I’m generally pro-evo devo throughout my course. I do try to make them aware of the bigger picture, though, so today we had an in-class discussion/’debate’ (nothing so formal as a debate, and it was more a tool to make them think about the arguments than to actually resolve a question). Fortunately, there’s one really easy exercise we can do in developmental biology, because some big names in the field have already clearly laid out their positions in a couple of relatively succinct papers, so I had a shortcut to bring the students up to speed on the issues. I split the class on Monday, having half read a paper by Hoekstra and Coyne on “The locus of evolution: evo devo and the genetics of adaptation” (pdf), which argues for the importance of trans-acting mutations in evolution, and another by Wray on “The evolutionary significance of cis-regulatory mutations” (pdf), which argues for the importance of developmental changes through changes in cis regulatory regions.
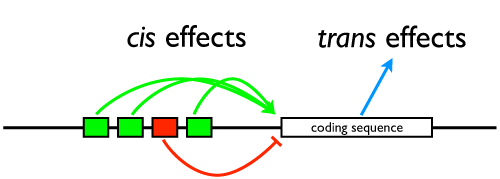
I drew this little cartoon on the board to illustrate the situation: that changes in the coding regions of genes produce mutations that can have broader effects throughout the cell (trans: they can affect other genes not on the same chromosome), while changes in regulatory DNA will have discrete effects on just the gene on the same strand of DNA they are (cis).
Then I asked them put together an argument as a group advocating for the significance to evolution of their ‘side’, cis or trans, which they then delivered to their opponent, with opportunities for rebuttal and counter-rebuttal.
Ah, pitting the students against one another…always the fun part of teaching.
There was good friendly discussion. Both sides had to dig into their respective papers to find the arguments, and then restate them to make their point, both of which are good exercises. The battle waged to and fro, and then our hour was up and I asked them to vote for who ‘won’, in the subjective sense of making a good argument and persuasively advancing their position. The results:
Which position do you think makes the best case for the significance of their phenomenon in evolution?
Team trans: 1
Team cis: 0
Both positions are important: 8
Minnesota mildness for the win!
I did think one student comment was perceptive and exposed the whole argument for a sham. If they were to go off to graduate school in developmental biology, they wouldn’t be picking Team trans or Team cis: they’d be pursuing a phenotype or a pattern of interest, and then analyzing how it worked and came to be, and they’d simply accept the evidence, cis or trans or both, however it turned out. Follow the data, always.
Now that’s a healthy attitude.