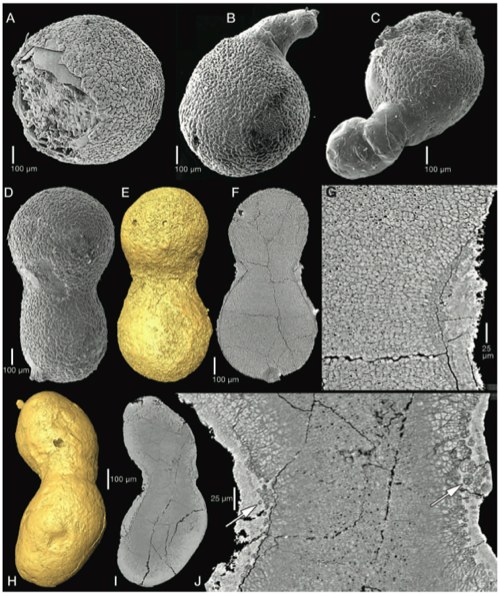
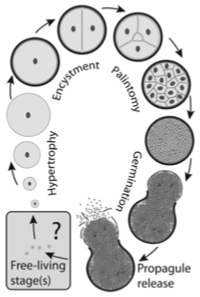
That most excellent blog on plant genetics and scientific agriculture, Biofortified, is having a fundraiser to maintain their educational and reporting efforts (there’s a full breakdown of where the money goes on the site). They’re not asking much and they produce so much more — help ’em out if you can.
(Also on Sb)