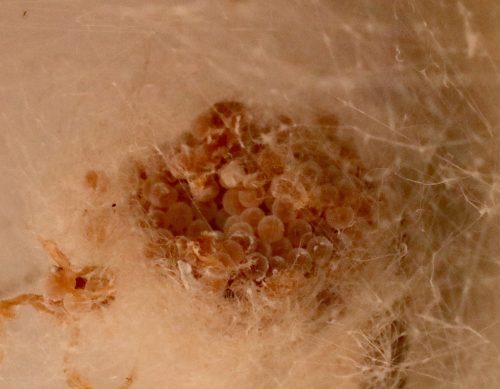
Isn’t technology wonderful?
Here it is, the aforementioned CT scan of a living spider egg sac (probably a theridiid of some kind)! 18 second scan taken at @BeckmanInst. I'll keep the sac to see if the babies hatch okay after x-ray exposure before releasing them back into the wild. https://t.co/uebbeD0lCC pic.twitter.com/9fnJdbtQ6l
— Josh Gibson, Ph.D. (@DrStrangeAnt) May 11, 2023
That reminds me, I have many balls of spiders to tend to this morning.