And they fluoresce, too!
These are part of a well-known invertebrate fossil bed, 22.5 million years old, in France. It contains lots of well preserved insects and spiders, and one question is…why? What makes this particular place so good at preserving these delicate specimens?
The fluorescence was a clue. They dug into the chemistry of the fossils, and figured out that the glow was produced by the sulfurization of chitin, that as the dead spiders sank in the diatom-rich waters of an ancient pond, the sulfur in the diatoms reacted with the chitin carbohydrate to produce a tough carbon polymer, inedible to the microbes, that could last for millions of years.

Cartoon shows the entire proposed pathway: spider becomes entrained in planktonic diatom mat. Pieces of the diatom mat, both with and without spiders entrained within fall to the sediment floor against a background sedimentation of other diatoms and algae (gray dots). With time, these sediments become compressed and preserved into the rock record. a Chemical composition of chitin. Two chains of chitin are illustrated, organized in anti-parallel. Gray boxes indicate the carbonyl functionalities on the chitin. b Sulfonate-containing molecule, which are common in diatom EPS, can undergo microbial sulfate reduction (MSR), leading to the production of sulfide. c Chitin molecule after sulfurization. C–S bonds could potentially replace the carbonyl functionalities, and S–S bridges could form across the chitin chains. d Idealized molecule representing a chitin polymer after further diagenetic alteration, which could result in the formation of aromatized carbon.
I thought that was kind of neat. It’s also a reminder to biology students that you never know where that organic chemistry we make you take might be useful.

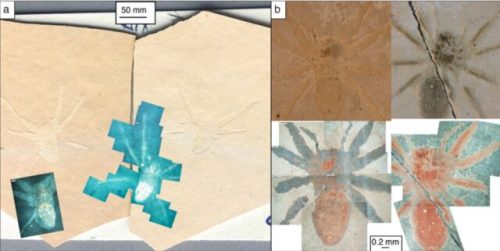

Sulfur cross-linking of chitin chains makes a natural polymer coating that preserved these fossil spiders for 22 million years.
As the biology prof says, you never know when that organic chem will be useful. Indeed!
What? And you’re saying this isn’t proof of an intelligent designer who intended us to see these fossils preserved for millions of years?
This is a nice example of something that obviously isn’t designed and also isn’t completely random either except for the chance circumstances that subjected the exoskeletons of spiders to this reaction. Instead, it’s a repeatable consequence of chemistry.
As humans, we can subjectively interpret it as enhanced “function”, namely to preserve the spiders’ bodies much longer than they would otherwise be. While that has no adaptive value to the spider, it is still very useful to present-day scientists. If a “designer” (non-omniscient) had been there 22.5 million years ago trying to figure out how to preserve these spiders for posterity, there is no reason to think they’d have come up with anything better than this chemical reaction.
I am forever baffled at people who see a “designer” behind every apparently functioning system. Reality is full of things that nobody intended but appear to work. You’d think anyone who ever stepped on a rake would get the basic idea. It is not limited to evolution.
Love it! More like this! Face the facts PZ, spiders are way more interesting when they are thoroughly dead.
So I guess all those piles of tires I see on the sides of secondary highways are gonna stick around for tens of millions of years? I guess after all the car bodies have rusted away, future archaeologists are going to find all these fossilized toruses and determine they were some sort of religious fetish objects, or possibly primitive money tokens?
Eukaryotes can be so tire-ing.
@moarscienceplz #4
When I was getting my degree in geology forty years ago and we were learning names of epochs eras periods etc. Many of these were marked by mass extinctions. we used to joke about this one being the Anthropocene because we were causing mass extinctions.
Nobody is joking now and there are serious proposals to change the name of the Holocene. If there’s any intelligent life on Earth a million years from now, the stratigraphy of death and toxins will be easily detectable.