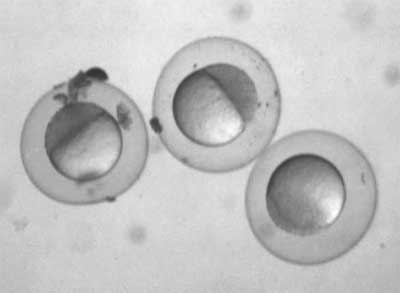
Oh, look: The first embryos from our new and improved fish system!
We only got a handful today, but you can see why. Those are about 3½ hours old, so we collected too late and the little babies’ mommies and daddies had spent the previous few hours assiduously poking around in the marbles sheltering the eggs, and had sucked up their little brothers and sisters in a cannibal feast, as they like to do. We’ll be adjusting our schedules, as developmental biologists often have to do, to do much earlier collections starting tomorrow.
The parents look happy and comfortable, perhaps a little plump after their breakfast of caviar and shrimp, so we expect more tomorrow. Right now we’re raising these little guys at a couple of different temperatures to calibrate our staging adjustments.