Note: the second figure has been replaced with the correct one, twice. A xylulose bond was in the wrong direction. This is hard.
There are a couple of other origin of life issues that I haven’t focused on yet. One that I was hoping would reveal itself is the issue of “chirality”, or the side of a molecule a bond or atom is on relative to the rest of the molecule in 3D. The strict definition includes things with identical chemical formulas having non-superimposable structures.
I don’t tend to draw in 3D to simplify things. I also don’t include hydrogen to simplify things.
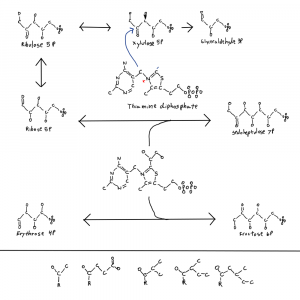
I meant to address this in a previous post where I posted this figure of thiamine and parts of the pentose phosphate pathway. It’s why there is a single hydroxal on xylulose-5P drawn in 3D as if it’s coming at you out of the page. I’ll get to that at the end.
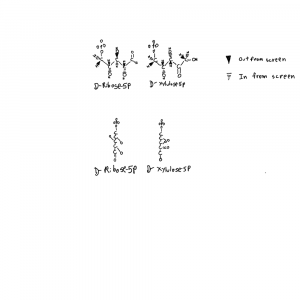
I’ve drawn ribose and xylulose in 3D. Both looking down the length of the molecule and from the side. Carbon actually binds 4 things and is tetrahedral like a pyrimid. So I put the hydrogens back into the upper structures and used conventional dashed and filled wedges to represent bonds going into and out of the plane of the page respectively.
Each of those hydroxals could be where the hydrogen is on the carbon instead. If you flipped the hydrogen and hydroxyl on ribose you can see how the formula would be the same but the structures would not be superimposeable. Things on different sides are designated with R/S or D/L distinctions that refer to single atoms on the structure. D-ribose refers to the stereochemistry around the atom farthest from the aldehyde group in the form of ribose used by life.
Amino acids are all L- relative to the side chains aside from glycine which has no atoms with stereochemistry.
So why is life one way and not the other when it comes to these bond directions? I don’t know but I noticed that the only sugar with a bond in the other conformation is xylulose-5P so I drew that one bond with 3D on the thiamine figure. The rest are in the other confirmation. And xylulose is the donor for the pentose phosphate pathway 2C molecules. This isn’t a solution to the chirality problem but it is a clue.

Isaac Asimov had an set of essays in his 1972 collection The Left Hand of the Electron on this subject (“Seeing Double”, “The 3-D Molecule”, and “The Asymmetry of Life”). And for all Asimov’s ego and other flaws he did have a doctorate in biochemistry, so this was very much his field.
So far as I know, the generally accepted answer to “So why is life one way and not the other when it comes to these bond directions?” is pretty simple: it may have been purely a 50-50 chance at first, but whichever chirality got the conveyor belt of life going first very quickly ended up producing the vast majority of most of these sugars and acids. And since so many of them don’t have any significant natural but non-biological production methods, the situation’s not going to change. Other planets where life evolved independently may have started with opposite chirality; we don’t know yet.
There may also actually be a connection between electron chirality and molecular chirality that would have tilted those initial chances away from 50-50, but we don’t know that for sure and it is really difficult to set up a test environment clean enough of any contaminants to actually validate this, because even a few pre-existing molecules of current sugars could bias any experiment.
Chemist here, love the posts so far! Not my forte, but origin of life chemistry is super exciting.
What I read of abiogenesis up to 2017, the chirality stems from the ancient parts of (pre)biotic synthesis. These were probably complex/chelating redox reactions. The freshly formed aminoacids were then acting as ligands themselves. The twist is: Chiral molecules that form complexes (for example with iron in iron sulfur minerals) imprint their chiral information on molecules that are synthesized by these complexes, because of steric hinderance. And so, as jenorafeuer said, once that process gets going, the chirality goes only one way because the chiral information is retained and which one “got chosen” by (chemical and thermodynamic) evolutionary processes was random.
Related are the origins of the bacterial and archaeal cell membrane lipids. They have different chiralities and liquid chiral polymers “de-mix” due to thermodynamic stability. So the ancestor made both chiral variants but due to instability of the mixed membrane, it evolved into two distinct membrane types.
I remember that in the 90’s and 00’s Wächtershäuser inspired some research on prebiotic synthesis and chirality and others picked that up. But these names just completely escape me right now and I don’t have my folders on hand.
I’ll have to look at electron chirality. I’m relearning thinking in moving electrons otherwise.
I’ve thought the left-hand rule in the relationship between magnetic and electric fields might be related, also anchoring and molecule and fluid going in one direction.
That’s interesting. I don’t suppose you remember the source for chelating reactions?
I’ll look into it when I have the time, sorry for now. Sadly I lost my researcher access to most papers, so a quick search gave me many paywalls.
The search keys might be “enantioselective synthesis”, “enantioselective complex catalysts”, an array synthesis methods that are used to selectivly make enantiomeres by using chiral catalysts. Carefully chemlated metal complexes are part of that.
This I found to be a useful overview that mixes chemistry and biology with good writing: https://pmc.ncbi.nlm.nih.gov/articles/PMC6396334/ maybe go on from their sources.
Btw. while looking I realized most chemistry papers on enantioselective synthesis look something like this (https://www.nature.com/articles/s41557-024-01473-5) and not too useful for the non-chemist (and many chemists tbh). Also I just found quite some creationist and asteroid-seeding-life’s-building-blocks papers on researchgate.
Sorry for the double posting but I feel the need to vent as I fell into rabit hole reading the shoddy work of a creationist organic chemist by the name Royal Truman while looking for good papers to link you. How he got a PhD in organic chemistry is beyond me.
“You are wrong, polyamide eating bacteria were created, no evilution, god did it”. Complete with citing Behe… . Dog darn. I need to lie down for a bit. I will not link it.
I’m sorry it took me a bit to approve this. I didn’t realize that it was caught by a filter. Thank you for posting these.
I am delighted to gain knowledge from your valuable expertise. Your generosity in sharing is greatly appreciated.
Optics plays an important role in many fields of science and technology. Understanding the problems related to optics not only helps to develop theory but also to apply it in practice.
The simple, melon sandbox pixelated art style is charming and perfectly suits the chaotic nature of the gameplay.
The game’s daily mode provides a Dordle single pair of puzzles available to all players at the same time.
xlope game is a simple yet addictive game where players control a ball rolling down an increasingly faster slope, dodging obstacles and chasms, and trying to travel as far as possible.
Geometry Dash continues to stand out in the platform game genre by offering a pure skill-based experience that relies on timing, focus, and consistency.