I think I poisoned my brain on Sunday reading these claims about different phases of water. Or, at least, I poisoned my Google algorithms because now this crap keeps gurgling up.
Here’s one that’s so over-the-top it was almost amusing, except that it’s a commercial site using ludicrous claims about biology to sell miracle water.
Dr. Gerald Pollack is a biomedical engineering research scientist from the University of Washington that discovered a new state of water beyond liquid, solid and vapor. H3O2, sometimes called gel water, structured water or exclusion zone water (EZ water), is in between a solid and a liquid. An extra hydrogen molecule and an extra oxygen molecule make it silkier than H2O. This matters because that 70 – 90% you’ve heard about in your body is actually H3O2. That’s why water doesn’t come gushing out of you like a hose if you get a cut. Your cells are full of the thicker, H3O2.
Oooh, silkier. How do they measure that? Also cool that they think I’d turn into a firehose if I only contained normal water.
Water in nature is naturally structured even though you can’t see any form in it. At a molecular level, under a microscope water has shapes that are organized in geometric patterns. Spring water, waterfalls and glaciers are structured. And the water in fruits and vegetables is naturally structured. What Dr. Gerald Pollack has revealed to us is that if we want to get our bodies into alignment with nature and health, we need to be thinking about hydration with structured water.
Yes. Put water under a microscope and you’ll be able to see the geometric patterns. I guess it’s supposed to look like this:
If your water looks nothing like that, you can buy a tube full of quartz crystals that will structure your tap water for the low, low, low price of only $1799.
Man, this is an amazing racket.

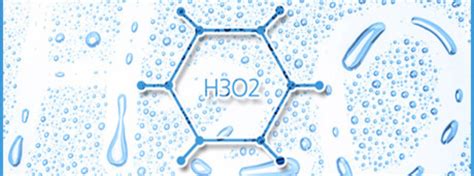

By silkier is he referring to Bombyx sori silk or arachnid silk? It’s vital to know if my vitals are more like spiders or caterpillars.
Well, my eyebrow is twitching. H3O2? I am guessing this would be a {ahem} reactive and short lived species (and no, I am not talking about briefly hydrogen bonded associations of H2O).
Louis
P.S. I refuse to comment on valency due to it exceeding my monthly swearword allowance.
No, no, it must be stable if 70-90% of the water in your body is in the H3O2 form, and if you can get it by just drinking water from a glacier.
I had a hard enough time when another wise entertaining fiction book had a supposedly tech savvy character refer to H2O2 as heavy water.
If it isn’t H2O then it isn’t water.
Oh, that’s it.
I’m going to start selling empty water bottles as Naturally Dehydrated Water. It’s dehydrated through a 100% natural process of evaporation and me drinking it, and can be safely reconstituted with water from any source because water remembers where it used to be. Or something.
OK, I’m still working on this, but really it’s just some marketing details that need to be worked out.
No cat, no cradle.
Well, water comes in 18 or so structured forms. All temperature and pressure related, of course.
All referred to as ice. Some forms being present in the mantle of this planet, which Gerald Pollack is encouraged to explore personally.
It’s been a long time since I’ve (consciously) done any chemistry, but I have a couple of doubts. Isn’t the chemical formula of water H2O, kind of definitionally? Wouldn’t H3O2 be a completely different substance, rather than just a different phase of water? Plus, I would assume that, given the water-plus formula, there are five atoms, while the “illustration” shows twelve, six with one available bond, six with three. Again, long time since, but doesn’t hydrogen have one available bond, while oxygen has two? I’m tempted to think H3O2 is best used for enemas, as that appears to be from where the doctor has pulled his research.
Correct me if I’m wrong, but the molecule shown is not even remotely water-like. It appears to be a molecule of cyclohexane, with a hydrogen stripped from each carbon. As such, it would be extremely reactive, ready to rob hydrogen from any other molecules it encounters, or to collapse into a benzene molecule as the open bonds attach to the neighboring carbon atoms.
It’s been over 30 years since I cracked an organic chemistry book, so maybe I’m off base on this, but I do know that if it ain’t H2O, it ain’t water.
Water, water everywhere. And these crackpots will hype their ‘magic water’ to anyone gullible enough to pay. How long have humans survived filled with only ‘generic’ water? I’m not too worried about water except for the fact that it’s being polluted everywhere by Corporations and that plentiful, clean water is getting scarce.
PZ said, “I poisoned my Google algorithms because now this crap keeps gurgling up.”
This proves that G00GLE does not provide objective results. It spies on you and feeds you what IT thinks you should see. ‘duck duck go’ still is much safer and yields more objective results, without all the spyware.
@Artor
If I’m being charitable, someone saw the crystalline structure for ice-1 and tried to draw a ‘molecule’ without any knowledge of ‘this is a three-D diagram and depth matters’ and ‘because this is a repeating pattern, all the edges have places for more water molecules to glom on’, and finally ‘it matters when drawing a molecule to mark which ones are hydrogen and which are oxygen, not to mention the difference between a hydrogen bond and covalent bond’.
Is H3O2 wet?
Man, this is an amazing racket.
Yeah, aside from the adware algorithm that brings the ads to the attention of someone who’s just shown his ability to debunk all their claims.
He has a future in the Republican Party. He is the first repent of the Emoto Peace Prize! Emoto is a Japanese charlatan who claims that human consciousness can alter the structure of water.
Okay, I’m looking at the chemical formula, and wondering about the structure. I mean, we all know water is H2O, and those of us who remember chemistry class will know that it’s arranged (“drawn” in linear fashion here, because typing) like this:
H-O-H
Because, as Richard Smith pointed out, H has one bond, and O has two.
You can add an O to the middle of the structure:
H-O-O-H
to get hydrogen peroxide, H2O2. You don’t want to drink that! You know how “antioxidants” are supposed to be good for you? Peroxide is the opposite. It is poisonous in low concentrations, and explosive in high ones. We do NOT have peroxide-based cytoplasm.
If Pollack was proposing H2O3, I could see how it was structured:
H-O-O-O-H
Yes, that is a real molecule, and no, that’s not in our cytoplasm either. I recommend this article (“Things I Won’t Work With: Peroxide Peroxides”) for more about that!
But he’s talking about H3O2. Where does that third hydrogen come in? There aren’t any free bonds to accept a single-bonded atom. Is he talking about hydrogen peroxide saturated with monatomic hydrogen (which would become diatomic as soon as the molecules bumped into each other)? Yikes!
I’d just like to point out that we as a species (and every other living thing on Earth) have been drinking ordinary water (or beverages based on it) for our hydration for as long as we’ve been here. Switching to something bizarre and expensive (not to mention poisonous!) would NOT be a good idea.
There are extreme cases of high-pressure phases of Ice that will exist on ocean worlds.
Having higher density than liquid water and existing at higher temperatures they will separate liquid oceans from the crust/ rocky mantle and starve such extra terrestrial deep oceans of minerals and other elements needed for life.
.
Fortunately there is no such phase of water as described in Vonnegut’s Cat’s Cradle.
But only if you’re Michael Jackson.
If you drink deuterated water (deuterium & oxygen) I am pretty sure it will be poisonous as it does not interact well with the ordinary water molecules in your body.
@29- Heavy water is 2 amu more massive than regular water, but the chemistry is very similar.
#13: It’s not wet, it’s silky.
Please, please search
“GAM 244 The Great Culling Our Water”
at Youtube. This is a dissection of one of the worst water based kook ‘documentaries’ you can find.
…and I have not even started in on the Flat-earther in England who washes with, and drinks his own urine.
PZ @ 21
Thanks for clearing that up. : )
Study Suggests Spins of ‘Brain Water’ Could Mean Our Minds Use Quantum Computation
Physicists mistakenly thinking they are biologists
Would make a great hair product if it existed.
Plus I believe animal starch (glycogen) stores most of our water. Go on a diet, it’s the first “weight” you lose rather than fat. It goes in the toilet.
BJ@19: “If you drink deuterated water (deuterium & oxygen) I am pretty sure it will be poisonous ”
It is, if in high concentrations- one study reckoned rats died when the D2O in their system exceeds 50%. But as for it not mixing with light water, well it does, that’s where they get it from.
BTW there are 8 different stable heavy waters, with one or two deuteriums and the three stable oxygen isotopes- oxygen 16, 17 and 18. You never seem to hear of the oxygen ones, even though there’s nearly 0.25% heavy oxygen in nature, and only about 0.03% deuterium.
Reginald Selkirk @25:
Non-physicists mistakenly thinking they have any idea what physicists are talking about, or that it has anything to do with the topic being discussed here.
$$$
Lies People Tell About Water – Part 3: Structured/Hexagonal Water, Water Memory
@27 — The toxicity of D2O, as you may know, is from the “isotope effect” in chemical kinetics, whereby reactions that require a D- bond to be broken proceed at a slower rate than for H-. This effect is greatest for D vs. H (since the mass ratio is 2x) and hardly noticeable for isotopes of heavier atoms like O. My understanding is that you’d need to drink many gallons of D2O (expensive!) over a prolonged period, to suffer ill effects from it.
Regarding Pollack, I’m curious how he came up with this H3O2 idea — I’ve no doubt that it’s wrong, but there must be a story that we’re not hearing here, cuz the guy does not strike me as a complete dummy. I would guess he means it as an empirical formula (of some oligomer) and not a simple molecule. An academic in any STEM field would have to know that “Show us your structure diagram” would be the first question to be asked at the seminar, so he must have one in mind. And I can’t believe that the picture in PZ’s post — 12 atoms? 18 atoms? in any case not H3O2 — came from Pollack or his group. If I’m wrong and it did come from him, well then, ok total crank, end of story.
What sort of chemical analysis he might have done, or thinks he has done, to come up with his formula, would be quite interesting to know. At least to me.
@birgerjohansson (#23): Nor have we discussed the mail-ability of said liquid… (I’ve listened to most of the “documentary” episodes more than once)
I have a further suggestion:
Fluorine is even better and silkier than hydrogen, why Teflon is a very silky feeling thing, that’s science that is. Therefore I propose this chap needs to play with FOOF.
A long, long way from where I am.
Louis
You really shouldn’t have looked it up. Over here I am bombarded with all sorts of Woo Water. Firstly there was oxygenated water then alkalised water. The latest magic snake oil is salt water. They take demineralised water, add a small amount of salt, stir in some outrageous health claims and sell it at a huge markup. The only advantage of drinking these overpriced tap waters is that it helps dilute the various snake oils that the gullible victims also swallow.
@16 That bugged me too.
I read the wikipedia page (https://en.wikipedia.org/wiki/Exclusion_zone_(physics)) on “the exclusion zone” (what this is obviously misunderstood from) and it had this paragraph:
I’m not sure what “bond” means in this context (not a chemist; never even had a college level chemistry class) but I think they mean a hydrogen bond (https://en.wikipedia.org/wiki/Hydrogen_bond). So I think rather than having a molecule with 3 hydrogen atoms they have two water molecules in which a hydrogen atom of one is attracted to (but not covalently bonded??? I don’t really understand all of it) to the oxygen atom of the other and… well, somehow they get three H and two Os but… well, I don’t know.
garydargan @ 33
Water with Uranium salt will give you more energy.
@35– …and superpowers.
Compared to this, polywater had all kinds of plausibility.
woodsong @16
Bridging hydrogens, like in boranes, but as part of an ozonide?
(I dunno why I’m even trying to make sense of this, as it’s obvious nonsense.)
Hydrogen ‘bonds’ are electrostatic bonds due to the polarisation of the hydrogen atom when it’s bonded to something like Oxygen. It is why water forms the crystals it does and why it expands below 4°C and when it freezes because it spaces out more. Because the oxygen becomes slightly polarised and the hydrogen does, one positively, one negatively. But it does not make the water not water and it is not H3O2.
They’re a lot weaker bonds than the covalent bonds between the hydrogen and the oxygen.
And yes you can get different structures of water ice under various different pressure/temperature conditions. But again, does not apply in this case.
Yeah, right, H3O2 is a new form water, just like CO is a new form of carbon dioxide.
Whatever happened to the good ol’ days when stupid cranks like this were just laughed at and forgotten? I don’t want to imagine someone like Velikovsky in the 2010s.
https://en.wikipedia.org/wiki/Hexagonal_water
Hexagonal water, also known as gel water, structured water, cluster water,[1] H3O2 or H3O2 is a term used in a marketing scam[2][3] that claims the ability to create a certain configuration of water that is better for the body.[4] The term “hexagonal water” refers to a cluster of water molecules forming a hexagonal shape that supposedly enhances nutrient absorption, removes metabolic wastes, and enhances cellular communication, among other things.[5] The scam takes advantage of the consumer’s limited knowledge of chemistry, physics, and physiology. Gel water is referenced in the version of the hoax in which plants or animal fascia are said to create or contain a “fourth phase” of water with an extra hydrogen and an extra oxygen,[6] despite the reality that this compound is neither water, nor stable—in other words it doesn’t exist in any practical sense.
Incompatibilities with science
The concept of hexagonal water clashes with several established scientific ideas. Although water clusters have been observed experimentally, they have a very short lifetime: the hydrogen bonds are continually breaking and reforming at timescales shorter than 200 femtoseconds.[7] This contradicts the hexagonal water model’s claim that the particular structure of water consumed is the same structure used by the body. Similarly, the hexagonal water model claims that this particular structure of water “resonates with energetic vibrations of the body to amplify life force”.[3] Although water molecules strongly absorb energy in the infrared range of the electromagnetic spectrum, there is no scientific evidence that supports the claim that hexagon-shaped water polymers would be created through bombardment of energy of these frequencies.[3]
Proponents of the hexagonal water model claim that the measurable differences[8] between commercially available “hexagonal water” products and tap water under 17O Nuclear magnetic resonance spectroscopy indicate hexagonal water’s special properties. However, this technique shows no significant differences between the supposed “hexagonal water”, ultrapure water, and human urine.[2][8] The experimental observation[9][10] of water clusters requires spectroscopic tools such as Far-infrared (FIR) vibration-rotation-tunneling (VRT) spectroscopy (an infrared spectroscopy technique).
https://cen.acs.org/articles/87/i50/Watering-Down-Science.html
“Such criticism might be career-damaging for some, but not so for Pollack. He is part of the first group of awardees for NIH’s T-R01 program, which aims to fund inherently risky, but potentially paradigm-shifting research (see page 29). Over five years, Pollack will get $3.8 million to pursue the “unexpectedly profound role of water” in biology and medicine.”
“Elizabeth L. Wilder, deputy director of NIH’s Division of Strategic Coordination, explains that the review process for the T-R01 grants “purposefully did not bring in people who were very close scientifically to each application,” she says. “The goal is to fund research that could break scientific dogma,” she tells C&EN, and including experts on the review panel “could be counterproductive.””
Welcome to the Twilight Zone.
My college library has Emoto’s books filed under “chemistry” and the librarians were furious when I suggested they be moved elsewhere. How dare I suggest censorship?
Out of idle curiousity, I googled H3O2. I don’t think I have ever seen the word “femtoseconds” used an a sentence before.