It’s in all the best webcomics!
I’ll take Paul Lynde for the center square, please. (oops, dated myself.)
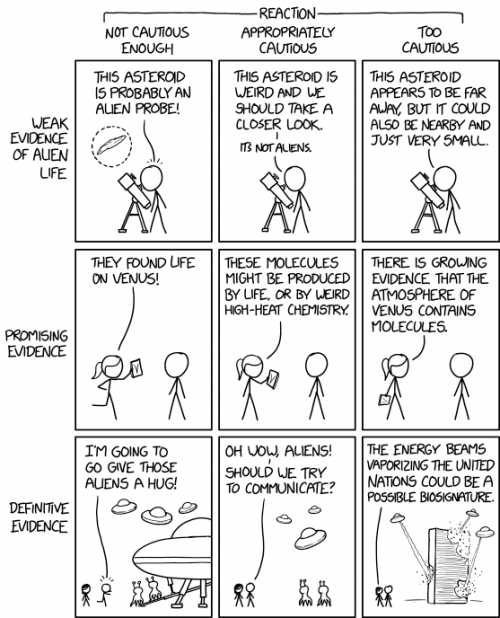
Finding phosphine in the atmosphere of Venus is interesting, and it sure sounds like the scientists who found it were all cautious and conservative and thorough, so I believe them that it’s there. I have a couple of caveats about how it’s interpreted, though. Phosphine can be produced abiotically — it’s found in the atmosphere of Jupiter, for instance — but it takes a lot of energy in reactions that weren’t thought to occur on Venus, but that could be the “weird high-heat chemistry” the comic mentions. It’s also produced biotically, on Earth by the decay of organic matter, but here it’s only a small component, and it’s also fairly rapidly broken down by sunlight. So we’re postulating huge quantities of organic matter decaying on Venus to produce phosphines, or an unusual organic process that produces persistent phosphines from a small quantity of biomass? I don’t know. Sounds unlikely and strange, but I love to see unlikely and strange.
No matter what, though, don’t expect Venusian cloud-cities and communicative aliens. Phosphine is a flammable, toxic gas, and at best we’re seeing the excretions of bacteria-like organisms, and even that is not likely.



Paul Lynde was a racist ass-hat.
“Phosphine can be produced abiotically”
I had to point that out immediately and took some flack for it. I want to believe too, but I consider Venutian life to be incredibly unlikely. As usual the media has gone for irresponsible sensationalism.
From the Wikipedia article (I know not a good source):
“Alternatively, the acid-catalyzed disproportioning of white phosphorus yields phosphoric acid and phosphine.”
There’s a lot of sulfuric acid in Venus’s atmosphere and we’re looking at parts per billion of phosphine. What I would like to see is the measurement of the ratio of phosphoric acid to phosphine. If the phosphine is higher than it should be then that would be an indicator that there is something really weird going on. As far as I can tell from this study, they only measured phosphine. That means they’re leaving half the equation out. The professor I learned o-chem from would be appalled if I turned in something like that.
— Phil Plait
Ray Ceeya @2:
I suspect the authors would’ve liked that too. There are probably limits to what they can detect (or infer) from the data they had. But it’s not as though they didn’t consider it;
Ok, not Venus related, but possibly cartoon worthy.
Help me out here. There’s much trumpeting in the news about irradiating people with far-UV to possibly kill Covid.
Does anyone else find this alarmingly premature and stupid?
stroppy @5: Maybe premature but promising?
Squee! Venusian bacteria!
I suppose that’s exciting. But in any case, Venus phosphine owes its existence to some chemical process, abiotic or not, since life itself is just another chemical process that happens to replicate its input. If we don’t go extinct first, I expect there will come a day when the discovery of yet another planet harboring “life” will be treated as mundanely as discovering a new species of insect here on Earth.
@5 stroppy
UV has never been a substitute for surface sanitizers. That bus boy at your favorite restaurant? That towel is soaked in some sort of surface sanitizer. Typically chlorinated bleach or quatrinary ammonia. Anything less than that would be unacceptable by health department standards. UV is far less effective than chemicals. TBH the UV stuff is more of a placebo in my mind than an actual solution.
Rob Grigjanis @ 6
I did notice something similar about the size of proteins. One of the things that bothered me was how things can morph from jazzed news coverage. I can see people getting confused and over exposing themselves to their detriment.
https://www.washingtonpost.com/health/sunlights-ultraviolet-wavelengths-have-strengths-limitations-in-disinfecting-against-the-coronavirus/2020/04/24/166c0816-7a67-11ea-8cec-530b4044a458_story.html
My first most immediate thought was WTF, aside from known UV damage from the sun, UV-C which normally gets filtered out by the atmosphere, grades into the x-ray part of the em spectrum. Speaking as someone who has had both skin cancer and cataracts, this gives me the willies. I also occasionally fiddle around with UV for photography and try to be informed about warnings and how to handle it, the general advice being better to be safe than sorry.
I guess if these were normal times I would probably be a little more sanguine about the possibilities. As it is I can see unwittingly walking into an establishment owned by some MAGA nut job blasting everything in sight with unfiltered ultraviolet.
UV rays sounds good got a touch of real science about it ,but what about them MAGA hat wearing wackaloons who don’t want to be exposed to them ,doesn’t the constitution say anything about big gubament forcing the people to cover themselves in UV.
If gog had wanted us to be covered in UV rays he would have done so already .
Will no-one think of them ?
I am now holding up a sign with sarcasm written on it .
stroppy @5 Far-UV might kill Covid (although inefficiently, as stated), but it also is absorbed efficiently by nitrogen and oxygen in air. It’s often called “the vacuum UV region”, as you must work with it in a vacuum if you wish it to irradiate anything with it. And yes, it is far deadlier than even UV-C.
As for phosphine on Venus, as an inorganic chemist I am completely unimpressed. At high temperatures and high pressures, everything is different. No reaction processes that are observed for phophine on earth are relevant. Reaction rates will be out-of-sight fast, and processes that never produce phosphine on earth could proceed rapidly.
I’d think that there’s not a lot of oxygen in Venus’ atmosphere? Since on earth, that is produced biogencially. That’ll be a huge difference, if true. And, if water vapor is in the atmosphere, it might be supercritical, for all I know. At that temperature and pressure, many gases might be, and liquids might not exist as we know them. Chemistry in supercritical fluids is completely different than in liquids or gases.
There might also be high insolation of high-energy radiation, since Venus is closer to the sun, although I don’t know how much is absorbed by the thick atmosphere.
Saying that “on earth this chemical is produced only biologically” is saying that “under the set of reaction conditions that exist in nature on earth, only certain reactions proceed.” Just like a chemist in a lab can change the reaction conditions and get different reactions (and I assure you that, by selecting the right conditions, I can make phosphine any time), when you change every single reaction condition to something that’s not just different, but extremely different than what’s on earth, you’re going to get extremely different reactions. I would be very surprised, in fact, if many reactions that aren’t found “naturally”, or only biogenically, on earth didn’t go quite readily in such different conditions as on Venus.
It’s like saying “I’m an ultra-high pressure, ultra-high temperature, completely anaerobic chemist who works in high concentrations of strong acid, but somehow I expect reactions in earth’s natural environment to resemble the reactions that I see.”
From what I’ve read, the authors are basically throwing up their hands with, “We can’t figure out how it’s being produced, but given the conditions at the cloud tops where we’re detecting it, life doesn’t make sense and no alternative source of the gas makes sense”.
Given the fact that the atmosphere of Venus at the altitudes the phosphine is being detected is “shirt sleeve” temperature and also a decidedly not “shirt sleeve” 75 – 95% sulfuric acid concentration, yeah, most certainly a touch ambiguous. Much akin to a certain “superluminal signal” eventually being found out to originate in dust in the fiber optic connections…
All, while the press takes what’s actually a question and runs with it as if it’s an ordained fact of the universe.
@12 wzrd1
“decidedly not “shirt sleeve” 75 – 95% sulfuric acid concentration”
LOL that’s one hell of an understatement. I used to work with bulk quantities of 95% sulfuric. That’s hazmat suit level work here on Earth. God I hated working with that system. You come in in the morning and one of the dosser pumps rotted through and the concrete containment was two feet deep with sulfuric. Pull on the respirator, grab a bag of bicarbonate and see how much you can get in before you start to run out of oxygen. If your fingers start to tingle, it’s time to leave.
wzrd1@12, yes, they are assuming that since phosphine is detected in the shirt-sleeve region, it must be synthesized there. Not the case: as with Jupiter, it could well have been synthesized abiotically in the completely unstudied and unusual conditions at or under the surface and have risen up.
They’re also assuming that the reaction of phosphine in the gas phase with gaseous sulfuric acid (or “clouds”, whatever those are) is the same as found on earth with aqueous sulfuric acid (“sulfuric acid” as we know it is an aqueous solution of SO2 gas in liquid water. About 18 moles of acid/liter dissolved in 55 moles of water/liter.) That’s never the case, with anything: water-solution reactions and gaseous reactions couldn’t be more different. The question is, how much water vapor is present in this region? Is the “sulfuric acid” gaseous or aqueous or what? They have to know before they draw conclusions.
Then, the atmosphere does contain a not-inconsiderable amount of SO2, which will drive the equilibrium of the reaction with sulfuric acid back towards phosphine, in any reaction medium.
So, they’d best study the thermodynamics and rates of the reactions that might actually take place, even in the shirt-sleeve region, before you compare them to reactions that happen on earth, and claim that phosphine must be quickly destroyed in the shirt-sleeve region and so be continual produced there by a biogenic process. Also, they’d better consider whether it’s being continually produced on or under the surface by conditions that don’t exist on earth, abiogenically. (For ex., at the surface conditions, CO2 is supercritical, and makes a great solvent, in which all kinds of unusual reactions occur.)
Center square not cautious enough, right hand square isn’t part of the same sequence (of course we know there are molecules – the announcement could only say “we have found a molecule previously unknown from Venus”). I would have preferred something like – “we have found a molecule new to Venus, in its clouds. On Earth this molecule can be produced by extreme physical conditions or by living organisms. Ockham’s Razor suggests it is being produced on Venus by extreme conditions.” But of course the media went for “Ooh Aliens!”
It probably isn’t life, though I don’t think it can be completely ruled out. Overall, what this shows is how much we don’t know about our nearest planetary neighbor. Venus seems to have been a bit understudied compared with our other planetary neighbor, Mars. I can understand why – conditions on or near the surface on Venus are so hostile that the probes that have landed there have only lasted for a few hours at most. Compared to Mars, where landers and rovers can last for years, the scientific return on investment from a Venus probe seems meager just because it won’t last long. I did read somewhere that current technology could probably make a Venus lander/rover that could survive up to a couple of weeks on the surface, and there are also plans for atmospheric probes, so there is still a chance for rewarding up-close Venus exploration, even though it presents lots of unique difficulties.
@garnetstar 14, my understanding is that there is no water present in the hellish atmosphere. That tends to make life problematic as well.
Given the fairly young surface and apparently planetwide remodeling from volcanic action, yeah, that phosphorus can pretty much come from anywhere, including outgassing during a never witnessed eruption (how would we even know if there was an eruption?). Given the CO2 pressure cooker atmospheric conditions, all manner of novel chemical reactions should be going on. I doubt that phosphates would manage to exist in that mess.
Venus: Where madness goes vacationing.
Jupiter: What happens on Jupiter stays on Jupiter, Jupiter insists.
SA had a fairly thorough and balanced piece on the report: Venus Might Host Life, New Discovery Suggests. The article also links to the actual report in Nature Science. It’s long and technical…way beyond me.
A big question, apparently is the evidence for phosphine at all. From what I gather it’s indicated by a single spectral line that’s either squiggly or fuzzy. But the authors have done repeated studies and think that the evidence that they are detecting phosphine is strong.
They do seem to have covered all the known ways it could be there and basically suggested a biotic process as a possibility along with unknown abiotic processes. Apparently there is some water in the Venus cloud deck where the phosphine is found, but it is sparse.
I gather the researchers are taking a cautious position and continuing to investigate. Obviously the best answer is the middle square no matter who’s sitting in it (there were a several others including Ernest Borgnine, Buddy Hackett, Wayland Flowers & Madame, and George Gobol).
@robro, I get that the researchers are at a quandary, understandably so. Traces of water aren’t sufficient to generate significant processes to generate a barely measurable signal.
So, it really still comes down to, “Hey, we observed this and can’t figure out howinhell it is consistently showing up.
I’m willing to bet that the authors and I would wait until some magical and otherwise invisible life form has its version of fangs thoroughly latched upon an ass cheek before we’d acknowledge its existence.
A rather similar report came out of CERN over a superluminal measurement of the velocity of neutrinos. Later, it turned out to be essentially dust in the fiber optic connectors.
Given that the surface pressure of Venus is 11 times what we experience and molten lead would be a norm, were lead on the surface, pressure does very interesting things to chemistry, as does heat, as does ionizing radiation at the shirtsleeves level of the atmosphere.
What it looks like to me is, a paper saying, “We don’t know what’s going on, can any of you figure this out, because we keep arriving at life, where we’re as sure that life is equally as possible as our turning into dragons.
Ran into a few of those problems and asked peers for help, which shocked them no end, then one or two found what I missed.
Even money, it’s a forgotten process of weathering, eventually making it up into the upper atmosphere.
Not Satan’s nut sack’s contents spewing magical life.
Europa, maybe, but I remain dubious, high radiation flux from Jupiter, colder than a a pair of brass monkeys…
Don’t get me started on Titan… There, as well as Europa, things turn into a reaction rate problem, as well as problematic conditions.
OT but I can’t resist…
‘colder than a pair of brass monkeys @wzrd1? I reckon you might get a kick out of this..
Back when the ships were wood (and the men were steel arrrhh!) in the Royal Navy and others they had to store the ammunition, the cannon balls, near the cannon. To do this they would make brass triangle frames called monkeys for some reason, fix those to the deck, and pile up the cannon balls in pyramids. Now, the triangles had to pretty tightly fit the cannon balls what the decks stubbornly refusing to stay level and still and all, but then again machining being what it was back then, with just a little bit of give to allow for errors there. Now when it got very very cold the brass would contract slightly more than the the cannon balls and all the cannon balls would fall off. Hence ‘Cold enough to freeze the balls off a brass monkey’.
A wonderfully imprecise yet very descriptive term. I love it. Source: I was a Royal Australian Navy sailor for 11 years and we have a lot of weird traditions and terminology that originated with the RN.
@robro, no, they haven’t covered all the known ways it could be there.
There’s a discussion of all the photochemical reactions (that they could think of) that could have produced it, which they say seem unlikely, and it is true that photochemical synthesis is the least likely route.
Then they say they considered all the non-photochemical ways too: that they “made a list of chemicals” that could produce it on the surface, and ruled out, by thermodynamic calculations only, synthesis of there.
But, in the Supplemental Data, where they claim to have listed all these non-photochemical ways that they considered, there’s only “to be published”. They don’t present their “list of chemicals” nor any reactions that they thought of. So, who knows what they considered!
Besides, calculation of the thermodynamic favorability of one reaction at a time, isolated from others, is not valid.
@indianajones, heard that story ages ago as well, even if I was US Army, joint operations introduced us all to various origin stories and traditions. :)
@garnetstar #21, indeed, as I recall, various asteroid associated meteor groups intersect the orbit of Venus, with some being associated with phosphorus bearing bodies. That’d be a far more likely origin than presuming life in an utterly anhydrous environment. Ionization of the upper cloud layers could trivially provide energy to associate and disassociate chemical bonding by UV alone.