I was talking about sex and nothing but sex all last week in genetics, which is far less titillating than it sounds. My focus was entirely on operational genetics, that is, how autosomal inheritance vs inheritance of factors on sex chromosomes differ, and I only hinted at how sex is not inherited as a simple mendelian trait, as we’re always tempted to assume, but is actually the product of a whole elaborate chain of epistatic interactions. I’m always tempted in this class to go full-blown rabid developmental geneticist on them and do nothing but talk about interactions between genes, but I manage to restrain myself every time — we have a curriculum and a focus for this course, and it’s basic transmission genetics, and I struggle to get general concepts across before indulgence in my specific interests. Stick to the lesson plan! Try not to break everyone’s brain yet!
But a fellow can dream, right?
Anyway, before paring everything down to the reasonable content I can give in a third year course, I brush up on the literature and take notes and track down background and details that I won’t actually dump on the students (fellow professors know this phenomenon: you have to work to keep well ahead of the students, because they really don’t need to start thinking they’re smarter than you are). But I can dump my notes on you. You don’t have to take a test on it and get a good grade, and you won’t pester me about whether this will actually be on the test, and you won’t start crying if I overwhelm you with really cool stuff. (If any of my students run across this, no, the content of this article will not be on any test. Don’t panic. Go to grad school where this will all be much more relevant.)
Onward. Here’s my abbreviated summary of the epistatic interactions in making boys and girls.
The earliest step in gonad development is the formation of the urogenital ridge from intermediate mesoderm, a thickening on the outside of the mesonephros (early kidney), under the influence of transcription factors Emx2, Wt1 (Wilms tumor 1), Lhx9, and Sf1 (steroidogenic factor 1). Even in the earliest stages, multiple genes interact to generate the tissue! The urogenital ridge is going to form only the somatic tissue of the gonad; the actual germ cells (the cells that will form the gametes, sperm and ova) arise much, much earlier, in the epiblast of the embryo at a primitive streak stage, and then migrate through the mesenteries of the gut to populate the urogenital ridge independently, shortly after it forms.
At this point, this organ is called the bipotential gonad — it is identical in males and females. Two genes, Fgf9 and Wnt4, teeter in a balanced antagonistic relationship — Wnt4 suppresses Fgf9, and Fgf9 suppresses Wnt4 — in the bipotential gonad, and anything that might tip the balance between them will trigger development of one sex or the other. A mutation that breaks Fgf9, for instance, gives Wnt4 an edge, and the gonad will develop into an ovary; a mutation that breaks Wnt4 will let Fgf9 dominate the relationship, and the gonad will develop into a testis (with a note of caution: the changes will initiate differentiation into one gonad or the other, but there are other steps downstream that can also vary). These two molecules may be the universal regulators of the sex of the gonad in animals: fruit flies also use Fgf and Wnt genes to regulate development of their gonads.
But the key to the genetic symmetry-breaking of selecting Fgf9 and Wnt4 varies greatly in animals. Some use incubation temperature to bias expression one way or the other; birds have a poorly understood set of factors that may require heterodimerization between two different proteins produced on the Z and W chromosomes to induce ovaries; mammals have a unique gene, Sry, not found in other vertebrates, that is located on the Y chromosome and tilts the balance towards testis differentiation.
Sry may be unique to mammals, but it didn’t come out of nowhere. Sry contains a motif called the HMG (high mobility group) box, which is a conserved DNA binding domain. There are approximately 20 proteins related to Sry in humans, all given the name SOX, for SRY-related HMG box (I know, molecular biologists seem to be really reaching for acronyms nowadays). SOX genes are found in all eukaryotes, and seem to play important roles in cell and organ differentiation in insects, nematodes, and vertebrates. Sry is simply the member of the family that has been tagged to regulate gonad development in mammals.
If a copy of Sry is present in the organism, which is usually only the case in XY or male mammals, expression of the gene produces a DNA binding protein that has one primary target: a gene called SOX9 (they’re cousins!). In mice, Sry is switched on only transiently, long enough to activate SOX9, which then acts as a transcription factor for itself, maintaining expression of SOX9 for the life of the gonad. Humans keep Sry turned on permanently as well, but there’s no evidence yet that it actually does anything important after activating SOX9; it may be that human males neglect to hit the off switch.
SOX9 binds to a number of genes, among them, Fgf9. Remember Fgf9? The masculinizing factor in antagonism to the feminizing factor Wnt4? This tips the teeter-totter to favor expression of Fgf9 over Wnt4, leading to the differentiation of a testis from the bipotential gonad.
So far, then, we’ve got a nice little Rube Goldberg machine and an epistatic pathway. Sf1/Wt1 and other early genes induce the formation of a urogenital ridge and an ambiguous gonad; Sry upregulates Sox9 which upregulates Fgf9 which suppresses Wnt4, turning off the ovarian pathway and turning on the testis pathway.
But wait, we’re not done! Sry/SOX9 are expressed specifically in a subset of cells of the male gonad, the prospective Sertoli cells. If you recall your reproductive physiology, Sertoli cells are a kind of ‘nurse’ cell of the testis; they’re responsible for nourishing developing sperm cells. They also have signaling functions. The Sertoli cells produce AMH, or anti-Müllerian Hormone, which is responsible for causing the female ducts of the reproductive system to degenerate in males (if you don’t remember the difference between Müllerian and Wolffian and that array of tubes that get selected for survival in the different sexes, here’s a refresher). Defects in the AMH system lead to persistent female ducts: you get males with partial ovaries and undescended testicles. So just having the Sry chain is not enough, there are downstream genes that have to dismantle incipient female structures and promote mature properties of the gonad.
As the gonad differentiates, it also induces another set of cells, the embryonic Leydig cells. We have to distinguish embryonic Leydig cells, because they represent another transient population that will do their job in the embryo, then gradually die off to be replaced by a new population of adult Leydig cells at puberty. The primary function of Leydig cells is the production of testosterone and other androgens. The embryo gets a brief dose of testosterone early that initiates masculinization of various tissues, which then fades (fortunately; no beards and pubic hair for baby boys) to resurge in adolescence, triggering development of secondary sexual characteristics. Embryonic testosterone is the signal that maintains the Wolffian duct system. No testosterone, and the Wolffian ducts degenerate.
Just to complicate matters, while testosterone is the signal that regulates the male ducts, testosterone must be converted to dihydrotestosterone (DHT), the signal that regulates development of the external genitalia. Defects in the enzyme responsible for this conversion can lead to individuals with male internal plumbing, including testes, but female external genitalia. Sex isn’t all or nothing, but a whole series of switches!
By now, if you’re paying attention, you may have noticed a decidedly male bias in this description. I’ve been talking about a bipotential gonad that is flipped into a male mode by the presence of a single switch, and sometimes, especially in the older literature, you’ll find that development of the female gonad is treated as the default: ovaries are what you get if you lack the special magical trigger of the Y chromosome. This is not correct. The ovaries are also the product of an elaborate series of molecular decisions; I think it’s just that they Y chromosome and the Sry gene just provided a convenient genetic handle to break into the system, and really, scientists usually favor the easy tool to get in.
One key difference between the testis and ovary is the inclusion of germ cells. The testis simply doesn’t care; if the germ line, the precursors to sperm, is not present, the male gonad goes ahead and builds cords of Sertoli cells with Leydig cells differentiating in the interstitial space, makes the whole dang structure of the testicle, pumping out testosterone as if all is well, but contains no cells to make sperm — so it’s reproductively useless, but hormonally and physiologically active. The ovary is different. If no germ line populates it, the ovarian follicle cells (the homolog to the Sertoli cells) do not differentiate. If germ cells are lost from the tissue only later, the follicles degenerate.
Ovaries require a signal from the germ line to develop normally. One element of that signal seems to be factors associated with cells in meiosis. The female germ line cells are on a very strict meiotic clock, beginning the divisions to produce haploid egg cells in the embryo, even as they populate the gonad. These oocytes produce a signal, Figα (factor in germ line a) that recruits ovarian cells to produce follicles. The male gonad has to actively repress meiosis in the embryonic germ line to inhibit this signaling; male germ cells are restricted to only mitotic divisions until puberty.
Even before Figα signaling becomes important, there are other factors uniquely expressed in the prospective ovary that shape its development. In particular, Wnt4 induces the expression of another gene, Foxl2, that is critical for formation of the ovarian follicle. The pathways involved in ovarian development are not as well understood as those in testis development, but it’s quite clear that there is a chain of specific genetic/molecular interactions involved in the generation of both organs.
Wait, you say, you need a diagram! You can’t grasp all this without an illustration! Here’s a nice one: I particularly like that cauliflower-shaped explosion of looping arrows early in the testis pathway.
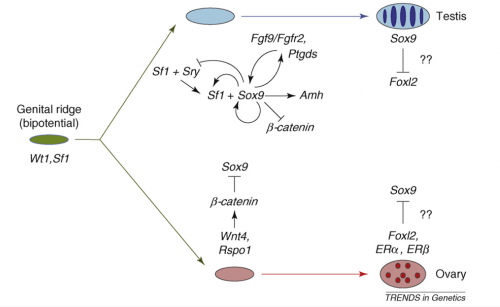
The molecular and genetic events in mammalian sex determination. The bipotential genital ridge is established by genes including Sf1 and Wt1, the early expression of which might also initiate that of Sox9 in both sexes. b-catenin can begin to accumulate as a response to Rspo1–Wnt4 signaling at this stage. In XX supporting cell precursors, b-catenin levels could accumulate sufficiently to repress SOX9 activity, either through direct protein interactions leading to mutual destruction, as seen during cartilage development, or by a direct effect on Sox9 transcription. However, in XY supporting cell precursors, increasing levels of SF1 activate Sry expression and then SRY, together with SF1, boosts Sox9 expression. Once SOX9 levels reach a critical threshold, several positive regulatory loops are initiated, including autoregulation of its own expression and formation of feed-forward loops via FGF9 or PGD2 signaling. If SRY activity is weak, low or late, it fails to boost Sox9 expression before b-catenin levels accumulate sufficiently to shut it down. At later stages, FOXL2 increases, which might help, perhaps in concert with ERs, to maintain granulosa (follicle) cell differentiation by repressing Sox9 expression. In the testis, SOX9 promotes the testis pathway, including Amh activation, and it also probably represses ovarian genes, including Wnt4 and Foxl2. However, any mechanism that increases Sox9 expression sufficiently will trigger Sertoli cell development, even in the absence of SRY.
So that’s what I didn’t tell my genetics students this time around. Maybe I’ll work it into my developmental biology course, instead.
Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. (2006) Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol 4(6):e187
Ross AJ, Capel B. (2005) Signaling at the crossroads of gonad development. Trends Endocrinol Metab. 16(1):19-25.
Sekido R, Lovell-Badge R (2009) Sex determination and SRY: down to a wink and a nudge? Trends Genet. 25(1):19-29.
Sim H, Argentaro A, Harley VR (2008) Boys, girls and shuttling of SRY and SOX9. Trends Endocrinol Metab. 19(6):213-22.
Yao H H-C (2005) The pathway to femaleness: current knowledge on embryonic
development of the ovary. Molecular and Cellular Endocrinology 230:87–93.


My, my, you do know how to get a body hot and bothered…
I’m here. I’m hooked. I can’t wait to learn. Back after finishing a reading with first impressions. Then I’ll try to really digest it.
Oh, I’m so excited when someone smart and knowledgeable goes off on an interesting topic!
I just took an exam on this! I’ve got a great lecturer right now but it’s always nice to read a different version. Helps it stick in my braincase better for the final.
PZ, one thing I would love to have covered in dev bio would be brainsex diversity between mammals and a class on human intersex conditions with a trans-inclusive bent. Issues of human sex, gender and sexuality seem like really fun topics to teach but have been entirely skirted in my three 400-level courses where it would have fit nicely, namely Dev Bio, Repro Bio and Endocrinology. Do you ever take any of these topics on in lecture?
So what happens if some mad professor genetically engineers an X chromosome with Sry on it?
Nice.
As far as I know, we still don’t know the mechanism by which temperature effects sex determination in those animals where it does.
Such information is, again as far as I know, entirely lacking. There would be nothing to cover.
That was a great explanation. Developmental biology is definitely not my expertise, but I love seeing how some of the same motifs appear repeatedly in biology. The gene circuits that lead to self-excitation and cross-inhibition with Fgf9 and Wnt4 operate on the same logic as neural circuits that are thought to play a role in categorical decision-making. If this is right, then in a certain sense, the developing gonad is “deciding” whether to adopt testicular or ovarian fate according to the same computational principles that we use to make perceptual decisions! (E.g., does that animal look like a cat or a dog?) Pretty cool.
But when you say it’s not inherited as a simple Mendelian trait…wouldn’t those generally involve triggering some series of regulatory genes too, even if it’s not always as complex a chain?
I really wish I could have coaxed you back to Norwescon this year: one of my panels in the Bio track is “Sex and Gender Fluidity,” which will look at how seemingly simple ideas like sex and gender are anything but. I may have to
stealborrow some of this material.Nope, haven’t seen a thing about the mechanism of temperature dependent sex determination. Phylogenetically, though, that initial step to break the Fgf9/Wnt4 balance is highly variable, and there’s probably a hundred different ways to break symmetry, and we’ll have to figure out a hundred different mechanisms.
In mice if you put an Sry gene elsewhere in the genome, you have complete reversion to male phenotype. There are also humans who are XX males because they have an Sry gene stuck somewhere it normally isn’t found.
ChasCPeterson@5:
Well, that depends on what you mean by “know the mechanism.” Quite a bit is known about various mechanistic factors that play a role in temperature-dependent sex determination in turtles, for example; this is practically an entire subdiscipline within herpetology. Just as an example, a quick Scholar search turned up this relatively recent paper, discussing a number of the same genes that PZ talked about above. Obviously we don’t know every step in the pathway, but that’s also true for mammalian sex determination.
That’s not entirely true either. My repro phys class in undergrad covered the mechanisms associated with intersex conditions in humans and other mammals; some of these are relatively well understood. An understanding of the mechanisms that may play a role in influencing gender identity or sexual preference is also not “entirely lacking,” but our understanding there is certainly more rudimentary, and focusing solely on the biological aspect of these biocultural traits in an undergrad class might be doing the students a disservice. But we certainly do have a reasonable grasp of how sex differences lead to the organization of different neural circuitry in some other mammals, and especially in birds, so this would be a perfectly cromulent topic for such a class.
In red-eared sliders (it’s a turtle, not a sandwich) the aromatase that produces estrogen from androgens is temperature-dependent. At medium temperature the aromatase makes enough estrogen for about 50% of the turtles to become female, whereas at extreme temperatures (really, just a couple degrees Centigrade in either direction) you get either 100% males or 100% females. You can cause all females by treating eggs with estrogen and you can cause all males by adding an aromatase inhibitor.
Here is a link on a recent article that talks about the molecular-level mechanism.
Fascinating! How much is known about the developmental systems in species in which individuals are hermaphrodites (either simultaneous or successive)?
I know. Let me be more specific: we still don’t know what step in the cascade is actually affected by temperature, nor how.
No it’s not. Read the abstract you linked. Aromatase expression (and therefore, of course, activity) is controlled by unknown temperature-dependent switches upstream.
Interesting to learn that differential methylation is somehow involved, though.
A very obvious comment, I’m sure, but… Whenever I learn anything like this, I’m always amazed that most of the time everything goes alright and that babies actually get born.
Also, it’s humbling to know that our bodies do all this stuff that we, with our minds and science, do not yet fully grasp. Our bodies “know” so much more than we do.
Which students? I think that by leaving the idea of brainsex unaddressed you probably leave people mired in superstitions and misconceptions about variations in gender and sexuality, and students who are sexual minorities are the ones who are done a disservice in that case. We know that in the mouse model adult sexual behavior can be entirely changed by stimulation with sex hormones in utero; why isn’t this the case in so many other mammals? What are the advantages and disadvantages of binary brainsex? These are interesting questions and would fit in nicely with information about the diversity of sex determination in animals, or with information on the strange phylogenetics of menses and estrous in the placental mammals.
In a 400 level class I expect to be asked to solve problems on exams in theory which are actually being approached by scientists in my field at the moment. There’s no reason why we shouldn’t ask students difficult questions about sexual behavior in mammals, and good reasons why we should.
I stand corrected. And now I am falling into a Google Scholar hole trying to figure out what aromatase inhibitor my prof was talking about here….
biogeo @6
Oooooooo ! Some good links please, please, please!!!!
Fascinating!
Any evolutionary explanation for why female germline cells meiotically divide at the earliest possible developmental time-point while male cells wait until sexual maturity?
Seconding Nick Gotts: How does this work for simultaneous (snails) or reversible (fish) hermaphrodites? Does anyone have a good answer on whether that happens via reversibility of these developmental pathways, or whether the gobies and whatnot are actually maintaining both sets and just turning on only one at a time?
#20
IIRC, female germline cells don’t divide after sexual maturity, which is why there is only a limited number of them – something to do with keeping the mitochondria as pristine as possible to be passed onto the offspring. Male germ cells have no need to preserve their mitochondria since they aren’t passed on.
Of course, I could be making all that up.
ChasCPeterson @14:
Fair enough.
Bicarbonate is back @18:
Unfortunately I don’t know a great summary offhand, but if you’re willing to slog through a pretty dense scholarly review, this review by Xiao-Jing Wang is pretty comprehensive of what we knew in 2008.
The basic idea, though, is you could imagine there are two sets of neurons, A and B (both of which include both excitatory and inhibitory cells — these could be two different brain areas, or just different subpopulations within the same brain area). A and B both project excitatory inputs onto themselves and inhibitory inputs onto each other, just like Fgf9 and Wnt4 each promote their own expression but inhibit each others’. A and B both receive excitatory input from various different sources, which you might think of as providing “evidence” to the two areas. Speaking very loosely, you could imagine that A are “cat cells” and B are “dog cells,” which let us categorize animals in front of us. Before we encounter the animal, neither population receives inputs, so we have no representation of “catness” or “dogness.” When we encounter the animal, we might see it has four legs and a tail, or pet it and feel that it’s fuzzy. These sensations are represented by various populations of neurons, all of which excite both the cat cells and the dog cells. The cat and dog cells also inhibit each other, but are balanced and so neither population gains the upper hand. But then the animal barks. The auditory neurons that decode barking project only onto the dog cells, so the activity of the dog cells increases above that of the cat cells. Now, because of the cross inhibition between the two populations, the dog cells inhibit the cat cells more than the cat cells can self-excite, and the activity of the cat cells is driven to zero, while the dog cell activity, no longer inhibited by the cat cells, soars, and we have arrived at a perceptual decision: this animal is a dog.
I should say of course that that’s a VERY simplistic explanation, and that this model has so far only been tested directly only for much simpler perceptual decisions than trying to categorize an animal as a dog or a cat. The actual neural computation for the categorization problem of dog vs. cat may not actually resemble what I just described, though this computation probably does play a role in at least some kinds of perceptual and behavioral decisions. But I hope it gives you a sense of the basic idea.
Anyway, you can see the similarity to the Fgf9/Wnt4 system. Prior to receiving inputs, they are in an unstable equilibrium, waiting to “decide” whether to trigger a testicular or ovarian fate. Upon receiving “evidence” from Sry or Figa (or temperature dependent mechanisms, or…), this equilibrium is broken and a “decision” is reached.
Do monotremes have it?
(5 X and 5 Y chromosomes…)
Probably a lot, because Caenorhabditis elegans consists of simultaneous hermaphrodites and males.
All I can say is Consider. The. Platypus.
Diagrams like that are conclusive proof that there is an intelligent designer, and he is Rube Goldberg on crack.
So there’s definitely a SOX in fox, then…
@David @ 24
Further evidence for my theory that the platypus is an evolutionary keyboard mash.
biogeo @ 23
Thank you for taking the time to write all that and refer to the article in question. What you wrote at 6 implies that we know a lot more than is in Xiao-Jing Wang and so I thought there was something new. I’m familiar with that literature but have been out of the game for about 5 years. It’s still mostly speculation and extrapolation.
Sox in a fox
Hox in a fox.
You will not find Sry in a fly…
…I’m trying to create a version of “Green eggs and ham” that goes “green eggs and sperm” but I don’t know any genes that rhyme with “Xenopus.”
@ biogeo thank you very much for dumbing it down. Falling down a new Google hole now.
WON’T SOMEONE THINK OF THE MENZ!?!??!?!
There’s no need to do this via genetic engineering; in some people the portion of the Y chromosome including Sry and several other critical genes is translocated across to the X chromosome by accident. If the person has the 46-XX karyotype the expression of the Sry gene usually results in the person developing masculine characteristics.
Page on the US National Library of Medicine (nlm.nih.gov)
I earned my PhD in the Capel lab (Blanche Capel), where your references are from! While my dissertation was on germ cell development and sex-specific cell cycle differences, I learned so much about sex determination. I performed several sex-reversal experiments where we generated male XX mice to determine the effects on cell cycle. My lab mate, Lindsey Mork, worked on sex-determination in the red-eared slider turtle. She published some awesome papers developmentally detailing the expression and importance of FoxL2. A post-doc at the time, Danielle Maatouk, has done amazing follow up work on the importance of Wnt4 and the female factors in establishing the female pathway. She recently accepted a faculty position at Northwestern to study chromatin regulation in sex determination. Another grad student at the time, Steve Munger, took a systems level approach to understand the entire genetic networks underpinning the process. Steve showed how some genetic backgrounds of mice which are predisposed to male-to-female sex reversal have higher expression of the gene networks that promote female development – it’s really amazing and interesting research!
Gender-related stuff in humans has my head in a vise, so I hesitated to read the comments here. But then I saw that shit about AWESOME ASS MONOTREMES and my heart is filled with joy. Next question – if their chromosomes which resemble the avian Z turn out to be due to a shared ancestor (rather than convergence), and that last common ancestor was some time in the Carboniferous, how amazing would it be that they conserved that complexity while the majority of extant cousins simplified? Even if they don’t have this stuff from that far back, how the heck did this situation evolve?
This is a fantastic subject.
Does this genetics course cover genomic imprinting at all? That could be an excellent topic allowing a return to discussing germ line development. I always find it pretty amazing that the imprints we pass on have to be established before we are even born. And there are of course mutants that help demonstrate the importance of maintaining imprints. In mouse you can delete Dnmt3l (DNA methyltransferase like gene only used in germ cells) and get viable pups. But the males are completely sterile, and females can only carry embryos for a little over a week. All due to a lack of parental imprints. In addition to imprinted genes, the female germ line has to deal with reactivating a silenced X chromosome. The resetting of imprints leaves germ cells vulnerable to transposon activity so you get dedicated defensive systems like the piRNA pathway.
Genetics and development of the germ line could be its own course.
We touch on imprinting and epigenetics in one lecture, near the end. It’s a challenge. These are smart students, but they struggle with just the simpler aspects of epistasis, so I have to ease them in. Genetics thinking isn’t trivial, but once you’ve done it for a while, it becomes natural…but they haven’t done it for a while yet.
sawells @26 took my comment in a different direction.
It would appear that Dr. Myers has conveniently proved irreducible complexity. Therefore Jesus.
Lately it seems the convention is arbitrary letter that is somewhere in the longform name + “ox”.
We’d better hope that there are no more than 26 distinct families of homeobox genes/proteins out there!
Or else our nomenclature systems will be totally screwed….
PZ, I got most of this in my 300-level Psychology of Women course, as introductory overview info on concepts of sex and gender necessary to establish what “Psychology of Women” is addressing in the first place, and it was on the test. You could totally go into this much depth in an actual biology course; or, at least, you *should* be able to do so.
Oh, my undergrad was at UW – Milwaukee; we’re not exactly hyperselective about who gets in as an undergrad.
This seems appropriate.
The second link doesn’t work, but the third leads to the paper – thanks!!!
(Spoiler: the platypus homologue of Sry is Sox3, which is on their chromosome 6.)
Whew, finally I have a free moment to comment. The morphology portions were a fascinating read, and I was happy I’d gotten things mostly right on the genetic side. Here’s my own characterization of the development process, written ten days prior:
There is no sex determiner at the genetic level, just a complicated froth of development pathways which is easily disrupted by a single mutation. The “sex chromosomes” aren’t even that important, as there are more sex-determining genes on chromosome 1 than there are on the Y ! Another source claims there are roughly thirty genes important for sex development, and only three exist on the X and Y chromosomes.
It’s no wonder we’ve retreated to simplified models to explain this mess.
Now that I think of it, this post also relates to an argument I had with Skep tickle, though I’ve heard many other people make the same claim: the default development pathway in mammals is female.
To argue something is the default is to argue it would happen without intervention, in this case via differential gene activation. But biology is nothing but differential gene activation! When you dig further into these claims, you find they all boil down into cherry-picking the evidence. Add a TDF gene to a 46,XX gamete that didn’t have a copy, and development proceeds along male lines. Don’t add it, and you usually get a female development process. Ergo, female is the default.
Which is great, until you realize adding a second DAX1 gene to an 46,XY gamete will lead to a female development process, even if TDF is present, and leaving a single DAX1 gene usually results in a male process. Ergo, male is the default! Myers himself presents a second argument for male-the-default above:
We can even make intersex the default pathway. It’s only after week five that the gonadal ridge forms, and sometime after week six that sex development starts. In other words, action is required to form any sort of gonad, and thus neither male nor female can be considered the default.
There simply is no “default” in biology.
If anyone’s curious, Skep tickle has argued against this. I personally don’t think it merits a reply, but other people might find her research interesting.
We haven’t. The “simplified model” – that people are either female or male – existed long before any of this mess was known. It persists, in everyday language and even in most areas of human biology, psychology, sociology and anthropology, because it’s a good, non-arbitrary, approximation: most people do end up either unambiguously male or unambiguously female. Denying that makes no more sense than denying that some people don’t.
Nick@46-“It persists, in everyday language and even in most areas of…”
Should it persist in everyday language? What do we achieve by branding every single human we meet with a male or female pronoun? I’d argue it’s a very bad thing. As for the rest, I’m not sure what in Hornbeck’s comment you’re disagreeing with. I’m far from an expert, but it seemed like they were just saying, “there is not ‘default gender’ developmentally, despite the cultural meme that it is female, derived from a simple view of XX=one thing XY=the other.”
Great American Satan @47:
After a few weeks of having people misrepresent me, despite multiple corrections on my part, having someone get my views right is a refreshing change!
Nick Gotts @46:
We already discussed that. If you want more, the discussion over at Fogg’s blog is little more than your argument repeated over and over and over and over and….
You’re welcome. :-)
Thanks for the outline and the link back to the kidney refresher. This sort of thing is the reason I originally started reading Pharyngula. Since you gave the name for Wt1, I was curious about the cause(s) of the Beckwith-Wiedemann Syndrome and its relation to Wilms tumors. If answering this is too messy, maybe there’s an online reference.
Sorry about reading this so late, but I’ve been out of town again.
Useless,
There are many sites with descriptions of the genetic changes related to Beckwith-Wiedemann Syndrome.
This NIH website is probably a decent starting point.
Basically the syndrome results from a range of different mutations that disrupt imprinting on chromosome 11. There are certain genes which only are active from the paternally or maternally inherited copy. Damaging or deleting the copy that is meant to be active leaves the individual with no functional version of that gene (even though the other parent’s copy is probably intact). BWS can also result from having 2 paternal copies of some of these genes and no maternal copy.
While there are multiple genetic changes that lead to BWS, several cause an increased risk for childhood cancers, including Wilms Tumor. The gene WT1 is located near the region of human chromosome 11 that is typically affected in BWS individuals. The site I linked above has more information on Wilms Tumor and other imprinting syndromes related to 11p15 disruption, such as Russel-Silver Syndrome.