Let us turn now to the One True God of science and atheism, the Ground State of our existence. We shall now contemplate Caffeine. Raise your cup in praise.
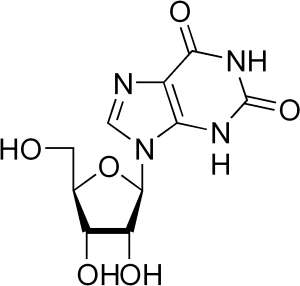
This morning, as I was sipping the blessed stimulant and browsing the journals, I learned a little something. I learned how caffeine evolved, and it was both unsurprising and surprising. I am not a biochemist, so it’s always enlightening to read a little out of that field. This, for instance, is the chemical precursor in the synthesis of caffeine.
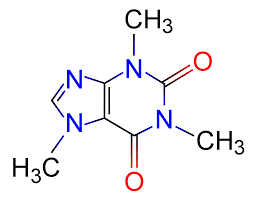
Oh, I said, I certainly do recognize that — I’ve been teaching budding cell biologists about important biological macromolecules for the past few weeks, and that’s a nucleoside. Note the ribose sugar with the attached nitrogenous base, the double ring of one pentagon attached to a hexagon? That’s xanthosine, which is also a precursor in the synthesis of those purines our cells use in signaling and assembling chains of nucleotides in DNA and RNA. That’s obvious. And then you look at caffeine, and it’s also obvious how cells make it: slap a couple of methyl groups (those -CH3s sprinkled around), and presto, you’ve got a happy drug. Easy.
Except…not all cells make significant amounts of caffeine. It takes specific enzymes to glue those methyl groups onto the core base with any reliable yield, and my cells might have xanthosine around, but they aren’t making any for me. I have to raid the vegetable kingdom and tear off their leaves and beans and hit them with boiling water to extract the blessed caffeine. And then not all plants make it — I can’t get a buzz on with a bowl of peas in the morning.
And then I learn that caffeine has evolved independently at least 5 times, and that the distribution of caffeine is patchy — some species in a genus will make it, while other related species in the same genus don’t. The caffeine molecule in Coffea (coffee) and Camellia (tea) and Theobroma is identical, but it’s synthesized by different pathways.
My mind is blown. Excuse me, but I have to take a break and think about this. I’m going to take a walk down to the coffee shop and gurgle down a couple of cups to help absorb this information. I’ll resume this post a little later.
You understand, don’t you? Go have another cup of coffee or tea while you’re waiting.
I have arrived at the coffee shop.
Must now worship reverently. Be back later.
The paper I’m reading is from PNAS, Convergent evolution of caffeine in plants by co-option of exapted ancestral enzymes, by Huang, O’Donnell, Barboline, and Barkman, and the title rather gives the story away. Here’s the abstract.
Convergent evolution is a process that has occurred throughout the tree of life, but the historical genetic and biochemical context promoting the repeated independent origins of a trait is rarely understood. The well-known stimulant caffeine, and its xanthine alkaloid precursors, has evolved multiple times in flowering plant history for various roles in plant defense and pollination. We have shown that convergent caffeine production, surprisingly, has evolved by two previously unknown biochemical pathways in chocolate, citrus, and guaraná plants using either caffeine synthase- or xanthine methyltransferase-like enzymes. However, the pathway and enzyme lineage used by any given plant species is not predictable from phylogenetic relatedness alone. Ancestral sequence resurrection reveals that this convergence was facilitated by co-option of genes maintained over 100 million y for alternative biochemical roles. The ancient enzymes of the Citrus lineage were exapted for reactions currently used for various steps of caffeine biosynthesis and required very few mutations to acquire modern-day enzymatic characteristics, allowing for the evolution of a complete pathway. Future studies aimed at manipulating caffeine content of plants will require the use of different approaches given the metabolic and genetic diversity revealed by this study.
So different plants have converged on synthesizing caffeine, using whatever enzymes they already had on hand, and by reconstructing ancient enzymes using the information in diverse modern sequences, they can uncover the actual steps taken to produce the modern, more efficient caffein production pathways.
Note that while I said it looks easy, just slap methyl groups on carbons in the rings, there are a lot of different ways that can happen. This diagram illustrates the great cloud of alternative intermediates — in the simplest sense, it’s a matter of the order that the three methyl groups are added.
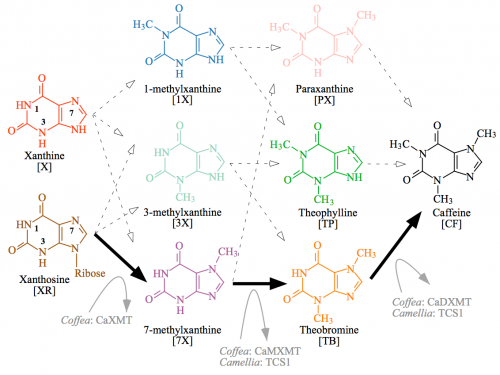
Caffeine biosynthetic network has 12 potential paths. The only path characterized from plants is shown by solid black arrows and involves sequential methylation of xanthosine at N-7, 7-methylxanthine at N-3, and theobromine at N-1 of the heterocyclic ring. Each methylation step is performed by a separate xanthine alkaloid methyltransferase in Coffea. In contrast, Camellia employs the distantly related caffeine synthase enzyme, TCS1, for both the second and third methylation steps, whereas the enzyme that catalyzes the first reaction remains uncharacterized. Other potential biochemical pathways to caffeine are shown by dashed arrows, but enzymes specialized for those conversions are unknown. Cleavage of ribose from 7-methylxanthosine is not shown, but may occur concomitantly with N-7 methylation of xanthosine. CF, caffeine; PX, paraxanthine; TB, theobromine; TP, theophylline; X, xanthine; 1X, 1-methylxanthine; 3X, 3-methylxanthine; 7X, 7-methylxanthine; XR, xanthosine
All of these intermediates can and will form naturally in the cell, but only the pathways that are facilitated by specific, effective enzymes will occur to a significant degree. So while methylation of nitrogens 1, 3, or 7 can occur in the first step, only 7-methylxanthine is produced because there is a specific enzyme, xanthine methyl transferase (XMT) that promotes that reaction.
In the second step, another methyl group is added to a different nitrogen, but different enzymes carry out this reaction in coffee (CaMXMT) and tea (TCS1).
Theobroma does it in a different order: the first step uses a CS1 enzyme to make 3-methylxanthine, and then a CS2 enzyme to convert 3-methylxanthine into theobromine in the second step. It’s madness! There are 12 different possible pathways to end up at the same final product, and it’s almost as if different plants plucked up different arbitrary enzymes to take a different path to caffeine.
One question, then, is where did all of these enzymes come from? After all, these are sequential pathways, and there’s no way to select for an enzyme to carry out the second step until an enzyme exists to carry out the first step, and there can be no selection for the first step in caffeine production until the whole pathway is present. This is the standard conundrum that utterly stumps creationists, and lead to the idea of irreducible complexity. How can you evolve a multistep pathway unless all the steps are selected for simultaneously?
And the answer is that all the steps use modified versions of other enzymes that carried out other functions in the cell. Plants maintained a methyl transferase for a 100 million years before the second step evolved because that primitive methyl transferase was facilitating other reactions in the cell, specifically in the methylation reactions needed for the production of benzoic and salicylic acid. And how do we know this? Because the investigators estimated the ancestral sequence of the enzyme from related species, made some of it, and discovered that it showed only weak activity in promoting methylation of xanthine, but much stronger activity with benzoic and salicylic acids.
The idea is that enzymes that performed one function had an incidental weak xanthine methyl transferase activity that generated enough 3-methylxanthine to act as a substrate for the second pathway to produce caffeine, which was sufficient activity to bootstrap selection for the whole sequence. The first enzyme could then be selected for increasing specificity for methylating xanthine to the point where the extant enzymes in some species are virtually completely specialized to produce 3-methylxanthine, and show negligible activity with benzoic or salicylic acid.
The answer, as I would have expected, is exaptation: a trait is coopted from one function to specialize it for another. It’s good to see another detailed molecular example of such an important idea, though.
It’s also good to see one of my favorite molecules showing up in multiple evolutionary trees.
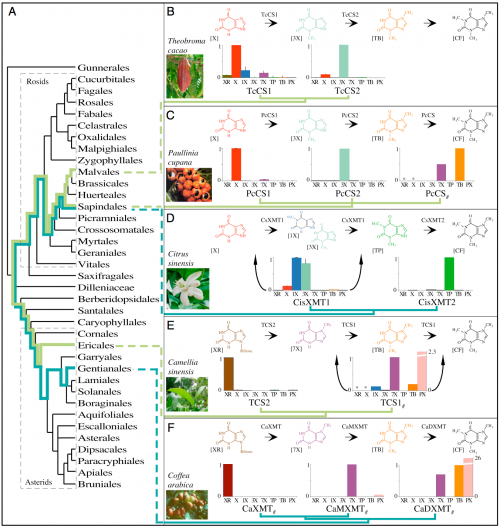
Caffeine has convergently evolved in five flowering plant species using different combinations of genes and pathways. (A) Phylogenetic relationships among orders of Rosids and Asterids show multiple origins of caffeine biosynthesis. Lime-green lineages trace the ancient CS lineage of enzymes that has been independently recruited for use in caffeine-accumulating tissues in Theobroma, Paullinia, and Camellia. Turquoise lineages trace the ancient XMT lineage that was independently recruited in Citrus and Coffea. (B and C) Theobroma and Paullinia have converged upon similar biosynthetic pathways catalyzed by CS-type enzymes. (D) Citrus has evolved a different pathway catalyzed by XMT-type enzymes, despite its close relationship to Paullinia. (E and F) Camellia and Coffea catalyze the same pathway using different enzymes. Proposed biochemical pathways are based on relative enzyme activities shown by corresponding bar charts that indicate mean relative activities (from 0 to 1) with eight xanthine alkaloid substrates. CisXMT1 and TCS1 catalyze more than one reaction in the proposed pathways. XMT and CS have recently and independently duplicated in each of the five lineages
Drink another cup and think evolutionary thoughts.



It is by caffeine alone I set my mind in motion.
It is by the beans of Java the thoughts acquire speed,
The hands acquire shaking,
The shaking becomes a warning.
It is by caffeine alone I set my mind in motion.
Warning: consumption may lead to the involuntary production of strange and dangerous tomes.
YOB: “It is by the beans of Java the thoughts acquire speed”
Caffeaist! Do you expect Camellians and Theobromaites to take that sitting down?
t is by caffeine alone I set my mind in motion.
It is by the leaves of China the thoughts acquire speed,
The hands acquire shaking,
The shaking becomes a warning.
It is by caffeine alone I set my mind in motion.
Did the coffee bean say to him that fashioneth it, how wakest thou?
So it’s not just me, life itself just can’t quit caffeine!
I see a lot of HO HO HO in that chemical diagram, plus a person with arms raised, holding the rest of the diagram in its left hand.
Ho Ho Ho
:Happy drug”
4sure
back to my cuppa
my o my,
looking again I see OH OH.
uh oh
back to my cuppa
Is it just me, or is the xanthosine hexagon ring upside down vs. the caffeine hex ring?
How does that happen? I get how the sugar group can get replaced, and how NH would get switched out for NCH3, but the carbon bonding changing around that much is confusing the hell out of me.
In today’s Grauniad, Fancy a brew? Celebrating the 358-year history of tea in the UK. Apparently, “On 23 September 1658, the London republican newspaper Mercurius Politicus carried the first advert for tea in the UK, announcing that a ‘China drink called by the Chinese, Tcha, by other Nations Tay alias Tee’ was available in a coffee house in the city.” (Today’s Generalissimo Google™ doodle apparently reflects this.)
The first coffehouse in London, however, dates to 1652…
Reference pretty please…
Where did all the extra come from…ignore my 11
I appreciate the pun, but it is, of course, a false
godvacuum. Gaze upon the true minimum of our potential, and despair! Also, cheers.I drink stimulants to rouse my brain to a sufficiently alert state to appreciate depressants.
#9: They’re just drawn that way. You can flip either molecule over and see they’re the same.
Wow, Java-juice seems to be good for STEM:
http://www.npr.org/sections/thesalt/2016/09/08/492954687/how-coffee-is-perking-up-engineering-education
I never drink coffee or tea. I do, however, eat chocolate-covered espresso beans, about which I am fervent.
So I count at least three denominations in the caffeinist religion.
1) The coffeeists, led by YOB (#1);
2) The teaists, led by Ice Swimmer (#4);
3) The beanists, led by me.
Eventually we will build rival seminaries, influence local governments, denounce each other as heretics, fight wars, and then make peace while still telling our children that the others are wrong. We’ll pay scientists to publish studies showing that other forms of caffeine worship are unhealthy. Politicians will look at polls and court whichever group has the strongest combination of numbers and political engagement in their districts. Maybe the teaists will have a disproportionate number of rich people, so eventually they will have a majority in Congress, with the result that national laws will favor them over . . . .
I can’t go on. Religion – any religion – poisons everything. Let’s all be thankful that caffeine is merely a commodity and not really an object of worship.
Sigh. All you coffee-drinkers and tea-drinkers! Outrageous hedonists, blathering on about the exquisite pleasures of plant materials steeped boiling water!
I’ve never enjoyed a cup of either, as I am a supertaster. The tiniest bit of bitterness (ie, coffee, tea, grapefruit, etc.) is quite intolerable. So I will calmly sip my cold water or soda while you moan and smack your lips and sigh.
Ah, the pleasures I will never know!
joel @ 17
Ah, but there are those of us who will happily be a part of all three denominations:
I’m a coffeeist every morning and often in the afternoon, with a shot of Kahlua added to my iced coffee.
I’m a teaist after swimming at the local pool or at the gym. Some days I even add a shot of Amaretto to my tea.
I’m a beanist for quick pick-me-up snacks or for nice decorations on my home-baked goods.
And here’s another denomination that no-one has mentioned – the chocolatists. I am happily a member of this sect – taking it in as either a liquid or a solid. I even like to mix denominations by adding chocolate to my coffee – mmm mocha on a cold day.
Your chocolate-covered beans are also proof that you cross denominations.
what do the plants that produce caffeine use it for?
@18 many sodas contain a lot of caffeine and they are not all cola.
uncle frogy
@Hairhead #18:
I’m afraid I struggle to imagine how that must be for you, but you have my sympathies. I couldn’t start the day without having a half or two of grapefruit while my mug of Earl Grey steeps, complete with its slice of lemon. Breakfast to me is having my mouth wake up before my brain needs to be nudged out of bed.
@Rich Woods 21
Thank you for the sympathy. This gets in the way of other things. I don’t drink alcohol because I can’t. The taste of alcohol is quite disgusting. In fact, I find the 0.5% alcohol in near-beer intolerable. I have looked for years for a mixed drink I could take in public, just to be sociable, you know. But, nope. Not a one.
@unclefrogy20
I love my soda, colas especially. The only time I had any kind of reaction to caffeine was when I tried one of those “monster” supercaffeinated fizzy drinks. Didn’t stimulate me, but made me feel a bit weird — it was unpleasant.
Stopped taking caffeine years ago. Like many other drugs you need the upper because it downed you when it wore off. Allows for much longer periods of concentration.
joel,
There are many paths to
enlightenmentembuzzment. We all must find our own way.Anyone know which version is usually used on soda? (And how does it get extracted prior to being put in soda?)
CC#25
Caffeine FCC (Food Chemical Codex). Origin, either extracted or synthetic, doesn’t matter.
Looking into the story I discover that citrus flowers are also a source of caffeine, about as strong as coffee. Fascinating!
unclefrogy@20: plants use caffeine mainly to ward off bugs, as I understand.
@ YOB +1 Dune reference
Dear PZ, this is the sort of article from you that I love. Caffeine evolved at least 5 separate times and is made by very different pathways? My brain hurts and now I need some coffee!
Make that at least six times; they left out yerba mate, which is apparently a holly (Ilex paraguariensis).
Turned out to be coffee, but it could have been coca.
Here’s a quiz designed to determine the taker’s Spirit Biological Macromolecule.
https://www.buzzfeed.com/commissioner/whats-your-spirit-biological-macromolecule-1ub8k
You’re welcome.
@Hairhead, Still Learning at 59, there is hope for you. I’m also a supertaster, but lost my sense of smell, allowing me to tolerate caffeine and still appreciate ethanol.
That said, I don’t recommend the condition, as I’ve had food poisoning more times than I care to relate.
Now, the question is, how to genetically modify my own genome to produce caffeine alongside of my diurnal hormones upon awakening… ;)
Although, it’d be likely be both easier and safer to modify a lowly orange tree to place caffeine inside of the oranges, giving me a boost of vitamin C, sugar and caffeine in the morning. :D
Yeah, it’d be an acquired taste. One that I’d be unlikely to come to appreciate.
Still, it never ceases to amaze me what one can build out of only four letters!
Or the sheer volume of convergent evolution out there in nature!
My question is not ‘How?’ but ‘Why?’; I mean, what advantage does production of caffeine impart on the plant such that selection favors the trait? (I think the ‘selectionist’ philosophy is warranted here because of the multiple occurrences.) Clearly, caffeine has positive (?) psychoactive effects on humans (pauses to take a sip), but does it (say) repel herbivores? Any clues?
@#9 medivh & PZ
Pretty much, but not quite accurate – it is worth noting that the double bond shifts from the 7 to the 9 nitrogen, as is seen more clearly in the biosynthetic network diagram. That is a function of the mechanism of the reaction, which proceeds from xanthosine to 7-methylxanthine via 7-methylxanthosine. The double bond effectively changes position when ribose is cleaved by a nucleosidase activity.
So plants evolved caffeine to prevent herbivores from feasting on their seeds… and yet now they fall prey to the opportunistic omnivore Homo sapiens. You’ll have to do better than that, plants!
Joke’s on you: Usefulness to humans is one of the most reliable indicators that you will always have watchers meticulously caring for you and spreading your seed on your behalf.
So teach us to number our days so that we may apply our harvesters unto coffee beans. Ramen, sister.
@Siobhan #37, point and match! ;)
Thank you for the inspiration: http://www.redbubble.com/people/michdevilish/works/23270837-coffee-chemistry?asc=u
Hairhead, have you ever played with miracle fruit? Miraculin binds to the taste receptors for bitter and sour, making everything taste sweet. It is delightful. I still dislike the flavor of coffee, but it makes the bitterness tolerable. (It also does amazing things for an IPA – I can taste all those fancy aromas people drone on about!)
Re @37/@37, Chili peppers are another (and perhaps more amusing) example. Originates in Mexico, but now found worldwide, entirely (and deliberately) because of long pigs.
What makes chili peppers so amusing is the chemical which makes them “hot”, capsaicin, seems to have been evolved as defense against mammals. Wild chili peppers depend on birds to spread the seeds (which pass through the ex-dinosaurs’s digestive system unmolested). Mammal’s teeth(?) damage / destroy the seeds, but mammals find capsaicin painful (birds do not), and hence — except for a certain hairless ape — don’t eat chili peppers.
Well, consider that excessive caffeine consumption even in humans can cause tachycardia – and then consider that we have considerably more body mass than a lot of small herbivores. Perhaps the biological function of caffeine to caffeine-bearing plants is to poison potential herbivore-predators by making their hearts tear themselves apart?
When I first learned that capsaicin was painful to mammals but not to birds, I immediately suspected it might’ve been “intended” that way (or less teleologically, peppers whose seeds only got eaten by birds and not by mammals made more plants), but I never looked up the science. Are you aware of any evidence that supports the hypothesis that it was adaptive? I’d love to be able to have a link to show people.